• Method: method validation
VIM
• Measurement procedure: procedure validation
GLP
• Standard operation procedure: SOP validation
Qc section
Karl Fischer titration Interview Related questions
Guide line
935 USP
Type Karl Fischer titration
Coloarimatry and volumetry titration
Colarimatry titration because use of a electromagnetic filed
Volumetric titration
There is a manually titration (classical method like In burette fill kf reagent and conical flask fill a Methanol see the end point straw yellow colour)
KF Reagent
It is a dark brown colour
Photosensitive
Moisture sensitive so after use cap would packed carefully
Store : Dry and dark place
Composite : Methanol,SO2,I2, pyridine or Imidazole
Reaction
SO2 + I2 + H2O = HI + SO3
SO3 + C5H5N = C5H5 N SO3
C5H5 N SO3 + C2H5 = C2H5 N SO3 C2H5
Volt use
15-20 V as on neutral
150 -200 V endpoint detection
Endpoint
Strow pale Yellow color
Indicator
Self indicator
Drift
Background Moisture call
Example vessel moisture
Role PH in Karl Fischer Reaction
Kf reaction depends on the pH value of the solvent. When pH is
between 5 and 8, the titration proceeds normally. However, when the pH is lower than 5,
titration speed is very slow. On the other hand, when pH higher is than 8, titration rate is fast, but
only due to an interfering esterification side reaction which produces water, resulting in an
vanishing endpoint. Thus, the optimal pH range for the Karl Fischer reaction is from 5 to 8, and
highly acidic or basic samples need to be buffered to bring the overall pH into that range.
More Q & A Found in given Link
Molarity
Definition :Molecular weight dissolved in 1000 ml solution called Molarity
Molarity Noted M
Formula Molarity
M = Gram of soult X 1000/
Molecular wt of soult X volume of soult
We have make 1M NaOH for 300 ml water
M = Gram of Soult X 1000/
40 X 300
Gram of Soult = 40 X 300
1000 X1 = 12 Gram
12 gram NaOH dissolve in 1000 ml water
If for use liquid (example
Use formula Density = M/V (Mass /Volume)
Eg For use H2SO4
V = 12/1.82 = 6.5934
6.6 ml H2SO4 in case
Why we need Molarity or use
Its needs because we have make different concentration solution in different places in such case
We have not identified Same concentration.
Principle TLC chamber
Chromatography works on the principle that different compounds will have different solubilities and adsorption to the two phases between which they are to be partitioned. Thin Layer Chromatography (TLC) is a solid-liquid technique in which the two phases are a solid (stationary phase) and a liquid (moving phase).
Use
Thin-layer chromatography (TLC) is a very commonly used technique in synthetic chemistry for identifying compounds, determining their purity and following the progress of a reaction. It also permits the optimization of the solvent system for a given separation problem
How the identify compound?
A quick TLC analysis can be used to identify whether or not an unknown compound is the same as another known compound. … If we find that the two spots have the same Rf-values, and the third spot only shows one spot, the two compounds are identical. The second common way to use a TLC- plate, is to monitor a reaction.
How do you prepared TLC Solution?
The choice solvent fraction for TLC start first low polar solvents then go to polar solvents. The solvent which gives maximum separation of the spot on TLC be selected. low polar solvents : pet-ether, n-hexane, Medium polar ethyl acetate, chloroform, DCM, High polar solvents methanol, ethanol, DMF etc
Which solvent good for TLC?
| Solvent | Polarity (arbitrary scale of 1-5) | Suitability |
| Water | 1 – Most polar | Good |
| Rubbing alcohol (ethyl type) or denatured alcohol | 2 – High polarity | Good |
| Rubbing alcohol (isopropyl type) | 3 – Medium polarity | Good |
| Vinegar | 3 – Medium polarity | Good |
Why hexane not good solvent?
Hi, It’s all about polarity matter. Hexane is a non-polar solvent, hence we usually start column from hexane and increase polarity slowly by using either ethyl acetate or DCM (it depends). Methanol is highly polar.
Why You can’t use a pen in TLC?
You can’t use a pen because the ink will travel up the TLC plate with the TLC solvent, just like your chemical samples do. You can only spot/label/mark TLC plates using a pencil. The graphite in a pencil will not run up the plate!
Mean of high Rf value?
A high Rf (Ie 0.92) would refer to a substance that is very non-polar. Ie that substance moved a 92% of the entire distance the solvent traveled. A low Rf value (0.10) would refer to a substance that is very polar
Calibration TLC Chamber
TLC Chamber are Calibrate Sodium Salicylate
At 254 , 366 nm
Limit
The lamp Should be capable pf revealing without doubt a standard spot of Sodium Salicyate with diameter of about 5 mm Cinematographic plate Coated with Silica gel
Explain lambert beer law
The Beer–Lambert law states that the quantity of light absorbed by a substance dissolved in a fully transmitting solvent is directly proportional to the concentration of the substance and the path length of the light through the solution
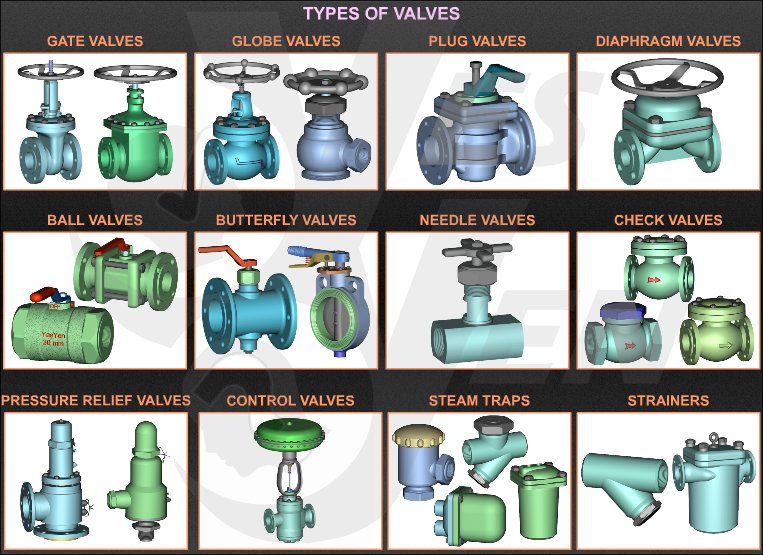
Type of valves
Ball Valve
Ball valve is a quarter turn operated valve. The closure member is a spherical plug with a through hole. When the valve is in open state, the through hole is in-line with the fluid flow and hence, the fluid passes through it. The valve is closed by rotating the globe by 90 Deg. such that the hole now becomes perpendicular to the flow and hence, stops the flow.
The seat is usually circumferential, made up of soft materials to offer a tight shutoff. The seat can be made either out of plastic or metals. Ball valves are not recommended to be used in a partially open condition. Due to misalignment between the flow direction and opening of the plug, large pressure drop takes place in partially open condition.
Due to above specified challenges, ball valves are mostly used in shutoff applications. Ball valves are commonly used in steam, water, oil, gas, air, corrosive fluids. They can handle slurries and dusty dry fluids. Ball valves are not used with abrasive and fibrous materials as it poses risk of damage to the seat and plug surface.
 Gate Valve
Gate Valve
Gate valve is a sliding type of valve. In gate valves, the closing member is a metal gate. The gate slides down to close the valve. In fully open conditions, the flow area is equal to the area of the pipe and hence, there is negligible pressure drop across the valve.
Gate valve should ideally be used as on-off valve. It is not advisable to use them as throttling valves because in partly open conditions, erosion of gate might take place. In partially open conditions, due to vibrations, valve is exposed to quick wear and tear. Also, during closing and opening, there is considerable amount of friction and hence, opening and closing these vales quickly and frequently is not possible.
These valves find their use in petrochemical industry due to the fact that they can work with metal-metal sealing.
Plug Valve
Similar to ball valves, plug valves are also quarter turn type of valves. This valve consists of a plug which can be either cylindrical or conical in shape. The plug has a through slit which remains in-line with the flow in the open condition. When the plug is turned by 90 Deg., this slit becomes perpendicular to flow and the valve gets closed.
Plug valves are well suited to handle fluids with suspended solids, slurries etc.
Plug valves are primarily used for on-off applications. When used for throttling purpose, the pressure drop through the valve is higher because of misalignment between flow direction and the direction of the opening (slit).
 Butterfly Valve
Butterfly Valve
Butterfly valves are most simple yet versatile valves. They are quarter turn operated valves which are commonly used in multiple industries for varied applications. Quarter turn operation ensures quick operating of the valve. In the open condition there is minimum obstruction to the fluid flow through the valve as the flow passes around the disc aerodynamically. This results in very less pressure drop through the valve.
Due to its unique mode of operation, the valve can be actuated easily without requiring high torques and wear and tear. Due to lack of friction, use of bulky actuators can be avoided. Another advantage offered by butterfly valve is their compact size. The valve is quite compact, resembling a metal disc. This makes their installation very easy. They can be used to handle slurries and fluids with suspended solids as there are no cavities for deposition of solid particles inside the valve body.
Globe Valve
Globe valve is a linear motion type of valves and is typically used in both on-off and throttling applications. In globe valves, the flow of the fluid through valve follows an S-path. Due to this, the flow direction changes twice which results in higher pressure drops. Due to other advantages offered by them, they are widely used in applications where pressure drop through the valve is not a controlling factor.
These valves are generally not used beyond sizes larger than NPS 12 (DN 300) as enormous forces are exerted on the stem to open or close the valve under fluid pressures. Globe valves require high pressures on the seat to keep it closed when the fluid exerts pressure from the bottom of the disc.
They are used for both on-off and throttling applications but special types of trims are required for throttling applications where large pressure drops are involved. These valves can be used in three configurations, depending upon the applications-
a. Tee pattern
b. Angle Pattern
c. Wye Pattern
When the disc is removed from the stem and allowed to rest on its own weight, globe valves can be used as non-return valves. Machining of seats is easier and cheaper compared to other types of valves.
Pinch Valve
Pinch valves consist of a plastic tube/sleeve which is made up of reinforced elastomers. The sealing/ closing action is achieved by throttling or pinching this sleeve/tube. Pinch valves are best suited for handling slurries and fluids having suspended solids. Pinch valves offer many benefits over the other types of valves. They can be used for handling corrosive fluids as there is no contact between the fluid carried and the actual valve mechanism. Once suitable sleeve material is selected, this valve can work with a variety of fluids. As fluid being carried does not come in contact with the metal parts, these valves can be used for food grade applications also.
Generally, pinch valves are suitable for low pressure applications. When used with abrasive slurries, they should be used as on-off valves; if used for throttling purposes, the sleeve will get worn out.
Disc Check Valves
Disc check valves, also called as non-return valves allow the flow to pass through them in only one direction and stop the flow in reverse direction. Because of this unique directional property, disc check valves are essentially used for some critical applications in the steam systems.
There are four major types of disc check valves as follows-
1. Lift Check Valve- Lift check valves work simply on the principle of gravity. When the fluid comes in the forward direction, the disc gets lifted from the seat against the gravitational force by the force of incoming fluid. The valve thus allows fluid to pass in this direction. When the fluid comes in opposite direction, it supports the force of gravity and the disc remains on the seat, keeping the valve closed.
Tight shutoff can be difficult to achieve in case back pressures are low. The valve will leak the fluid in such situations.
 2. Swing Check Valve- In this kind of check valve, the disc or the closing element swings around a point to which it is hinged. When the fluid comes in the forward direction, the disc swings in an open position allowing the fluid to pass. When the fluid flow comes in the opposite direction, the disc swings and rests on the seat to lose it.
2. Swing Check Valve- In this kind of check valve, the disc or the closing element swings around a point to which it is hinged. When the fluid comes in the forward direction, the disc swings in an open position allowing the fluid to pass. When the fluid flow comes in the opposite direction, the disc swings and rests on the seat to lose it.
3. Spring loaded Check Valves- In this kind of check valves, tight shut-off it provided using a spring. The spring holds back the disc on the seat. Even in the forward flow condition, the fluid should exert some pressure, called cracking pressure in order to open the disc against the spring pressure.
4. Diaphragm Type Check Valve- This kind of check valves uses diaphragms arranged in such a way that that open to allow the flow only in forward direction. When flow comes from the reverse direction, the diaphragms remain closed.
Typical applications in a steam system-
1. After a float trap- Steam traps are passive device and work on the principle of the pressure difference. During operation, process pressure might go under the backpressure after trap. In such situations, because of the negative pressure across trap, condensate might go back into the process equipment through the trap. Hence, it is always advisable to fix a disc check or non-return valve after the float trap. This check valve will allow the condensate to flow from the trap outlet to the condensate recovery system but will ensure that it does not flow in reverse direction.
2. Mixing applications- Applications where two or more fluid are mixed, check valves should be installed at the end of each individual line. This avoids the contamination of one fluid by the other.
3. Disc check valves as vacuum breakers- Disc check valves, when fitted in a reverse way, can act as vacuum breakers. While being used as vacuum breakers, they should be used as shown in the figure below-
Under normal operating conditions, the valve will remain closed not allowing the steam to pass through it. When the vacuum formation takes place (during shutoff) the disc will open and will allow the air to come in thus avoiding the formation of vacuum.
What is EDQM ?
European Directorate for the Quality of Medicines & HealthCare.
GUIDELINE FOR RESIDUAL SOLVENTS
Q3C(R8)
buffer solution
A buffer solution is an acid or a base aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it
What is call Liniarity
The linearity of an analytical procedure is its ability (within a given range) to obtain test results which are directly proportional to the concentration (amount) of analyte in the sample.
Compartment of HPLC & GC ?
HPLC
Going into more detail, HPLC consists of a variety of components, including a solvent delivery pump, a degassing unit, a sample injector, a column oven, a detector, and a data processor.
GC
GC uses an inert or unreactive carrier gas as the mobile phase, and the stationary phase is generally a thin layer of liquid. As the mobile phase moves, it separates the mixture into its individual components in the stationary phase
What is call Mass spectroscopy
Mass spectrometry (MS) is an analytical technique that separates ionized particles such as atoms, molecules, and clusters by using differences in the ratios of their charges to their respective masses (mass/charge; m/z), and can be used to determine the molecular weight of the particle
What is the most useful range of IR?
between 4000 – 670cm–1
Give name reaction
- Aldol Condensation: We can define aldol condensation as an organic reaction in which an enolate ion reacts with a carbonyl compound to form β-hydroxy aldehyde or β-hydroxy ketone, followed by dehydration to give a conjugated enone.
- Balz-Schiemann Reaction: The Balz-Schiemann Reaction is named after German scientists Günther Schiemann and Günther Balz. The reaction showcases the conversion of arylamines to aryl fluorides. The reaction happens through diazotization after which thermal decomposition of the derived hexafluorophosphate or tetrafluoroborates. Visit Balz-Schiemann Reaction for more information.
- Cannizzaro Reaction: The Cannizzaro Reaction is named after Stanislao Cannizzaro. The chemical reaction signifies the base-induced disproportionation of a non-enolizable aldehyde. Click here for more information on Cannizzaro Reaction.
- Clemmensen Reduction: The Clemmensen Reduction reaction is named after a Danish scientist, Erik Christian Clemmensen. The Clemmensen Reduction reaction shows how to reduce ketones or aldehydes to alkanes using zinc amalgam and hydrochloric acid.
- Etard Reaction: The Etard Reaction is named after a French chemist named Alexandre Léon Étard. The Etard Reaction is a chemical reaction in which a heterocyclic bound or an aromatic methyl group is directly oxidized to an aldehyde using chromyl chloride. Visit Etard Reaction for more information.
- Finkelstein Reaction: The Finkelstein Reaction is named after Hans Finkelstein, a German chemist. The Finkelstein Reaction deals with the exchange of a halogen atom by a Substitution Nucleophilic Bimolecular reaction. Click here for more information on Finkelstein Reaction.
- Friedel Crafts Reaction: Friedel Crafts Reaction was developed by Charles Friedel and James Crafts in 1877. The Friedel – Crafts Reaction is a way of attaching substituents to an aromatic ring. Visit Friedel Crafts Reaction for an in-depth explanation of the reaction details and mechanism.
- Gabriel Phthalimide Synthesis: Gabriel Phthalimide Synthesis was discovered by a German chemist named Siegmund Gabriel. The Gabriel synthesis is a chemical reaction used to obtain primary amines from primary alkyl halides.
- Gattermann Reaction: The Gattermann Reaction is named after a German chemist, Ludwig Gattermann. Also known by the name, Gattermann salicylaldehyde synthesis is a chemical reaction in which aromatic compounds in the presence of a Friedel–Crafts catalyst are formylated by hydrogen cyanide.
- Gattermann – Koch Reaction: The Gattermann–Koch reaction is named after the German chemists Julius Arnold Koch and Ludwig Gattermann. In a Gattermann–Koch reaction refers to a Friedel–Crafts acylation reaction in which a Friedel–Crafts catalyst, hydrochloric acid, and carbon monoxide are used to produce aromatic aldehydes from various aromatic compounds, including derivatives of naphthalene and benzene. Click here for an in-depth explanation of the reaction details and mechanism of Gattermann – Koch Reaction.
- Grignard Synthesis: A Grignard Synthesis Reaction is a chemical reaction in which vinyl, alkyl, or aryl-magnesium halides (Grignard reagents) add to a carbonyl group of a ketone or aldehyde. Visit Grignard Synthesis for more information.
- Kolbe’s Reaction: Kolbe’s Reaction is a chemical reaction where aromatic hydroxy acid is produced from the heating of sodium phenoxide with carbon dioxide under a pressure of about 100 atmospheres and the successful treatment of the resulting product with sulfuric acid. Click here for an in-depth explanation of the reaction details and mechanism of Kolbe’s Reaction
- Reimer- Tiemann Reaction: Reimer- Tiemann Reaction was discovered by Karl Reimer and Ferdinand Tiemann. They have a private track to test the used for the ortho-formylation of phenols. Visit Reimer- Tiemann Reaction for more information.
- Rosenmund Reduction: The Rosenmund reduction was named after Karl Wilhelm Rosenmund, who in 1918 reported its discovery. A hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde.
- Sandmeyer Reaction: The Sandmeyer Reaction was named after Traugott Sandmeyer in 1884. Sandmeyer Reaction is a chemical reaction in which we use aryl diazonium salts to synthesize aryl halides. Sandmeyer Reaction provides the capability of performing some unique transformations to benzene such as hydroxylation, trifluoromethylation, cyanation, and halogenation.
- Stephen Reaction: Also known by the name, Stephen aldehyde synthesis, was discovered by Henry Stephen. This chemical reaction involves the preparation of aldehydes from nitriles using tin(II) chloride, hydrochloric acid and quenching the resulting iminium salt with water. During the synthesis, ammonium chloride is also produced. Click here for an in-depth explanation of the reaction details and mechanism of Stephen Reaction.
- Swartz Reaction: Swarts reaction and Finkelstein reactions are halogen exchange reactions that are associated with alkyl halides. Visit Swartz Reaction for more details.
- Williamson Synthesis: This a chemical reaction to implement ether synthesis discovered by Alexander Williamson in 1850. The Williamson ether synthesis is a chemical reaction for forming an ether from an organohalide and deprotonated alcohol.
- Wolff – Kishner Reduction: The Wolff – Kishner Reduction reaction was first reported by Nikolai Kishner in 1911 and Ludwig Wolff in 1912. This is a named reaction in organic chemistry involving obtaining methylene groups by converting carbonyl functionalities. Click here for an in-depth explanation of the reaction details and mechanism of Wolff – Kishner Reduction.
- Wurtz Reaction: The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium metal in dry ether solution to form a higher alkane. Visit Wurtz Reaction for more information.
- Wurtz – Fittig Reaction: The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Click here for more information on Wurtz Fittig Reaction.
- Carbylamine Reaction: The Carbylamine reaction is also known as Hofmann isocyanide synthesis. It is the reaction of a primary amine, chloroform and a base to synthesize isocyanides. Click here for an in-depth explanation of the reaction details and mechanism of Carbylamine Reaction.
- Fischer Esterification: Fischer Esterification is an organic reaction which is employed to convert carboxylic acids in the presence of excess alcohol and a strong acid catalyst to give an ester as the final product. Visit Fischer Esterification for more information.
- Haloform Reaction: The Haloform reaction is a chemical reaction where haloforms are produced by the exhaustive halogenation of methyl aldehyde or methyl ketone with a base present. Click here for more information on Haloform Reaction.
- Hell-Volhard-Zelinsky Reaction: The Hell-Volhard-Zelinsky reaction is used for the halogenation of carboxylic acids at the alpha carbon. Click here for an in-depth explanation of the reaction details and mechanism of Hell-Volhard-Zelinsky Reaction.
- Hoffmann Bromamide Reaction: The Hoffmann Bromamide reaction is used for the conversion of a primary amide to a primary amine with one less carbon atom.
Different between iodimatry and iodomatry titration
Iodometry is a titration in which the reducing agent is utilised to titrate the iodine formed in the preceding redox reaction, whereas iodimetry is a titration in which the reducing agent is used to titrate the iodine solution directly.
back titration
A back titration is a titration method where the concentration of an unknown compound is determined by reacting with a known amount of excess reagent. A back titration is also called indirect titration. There is a chemical reaction between these compounds.
Check out “Pharma Interview”
Method validation USP chapter<1225>
Aurobindo Pharmaceutical Limited|| USFDA give 483 ||AUGUST- 2022
Aurobindo Pharma on Tuesday said the US health regulator has issued Form 483 with three observations after inspecting its manufacturing facility at Pydibhimavaram in Andhra Pradesh.
As per US Food and Drug Administration (USFDA), Form 483 is issued to a firm’s management at the conclusion of an inspection when the investigator has observed any conditions that in its judgement may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts.
The Pydibhimavaram unit was classified as OAI (official action indicated) on May 17, 2019, and subsequently given a warning letter on June 20, 2019 by the USFDA, Aurobindo Pharma said in a regulatory filing.
Subsequently, the company said it has responded to the warning letter and carried out the committed corrections and the USFDA authorities inspected the facility from July 25 to August 2, 2022.
”At the end of the inspection, we have been issued a Form 483 with three observations and none of these observations are related to data integrity,” Aurobindo Pharma said.
The company will respond to the USFDA within the stipulated timeline and work closely with the regulator to address the observations at the earliest, it added.
The unit is a non-antibiotic active pharmaceutical ingredients manufacturing facility.
Links observation